Chemical Inventory & Storage
Maintaining an accurate chemical inventory is both a regulatory requirement for using, handling, and storing hazardous materials as well as a cornerstone for effectively communicating and managing chemical hazards present in the laboratory.
It is primarily the Departments’ responsibility to ensure that proper handling and storage protocols are being followed in order to reduce the risk of unnecessary exposure to potentially hazardous substances.
Enterprise Risk Management (ERM) can provide additional resources and guidance to help laboratories prepare effective and comprehensive inventories of the hazardous chemicals present in laboratories. The use of tools such as the online SDS database are encouraged by ERM to help with inventory management. Ensuring that this information is frequently updated is the responsibility of the Department. However, ERM, as part of the annual Departmental Chemical Hygiene Plan (DCHP) review can help to update outdated inventory. It is extremely important that provided chemical inventory information is current so it can be effectively interpreted to ensure accurate reporting to relevant regulatory entities and is available for first responders when necessary. ERM additionally helps laboratories to effectively leverage chemical inventory information to make safe and effective choices in the handling, storage, and overall management of laboratory reagents.
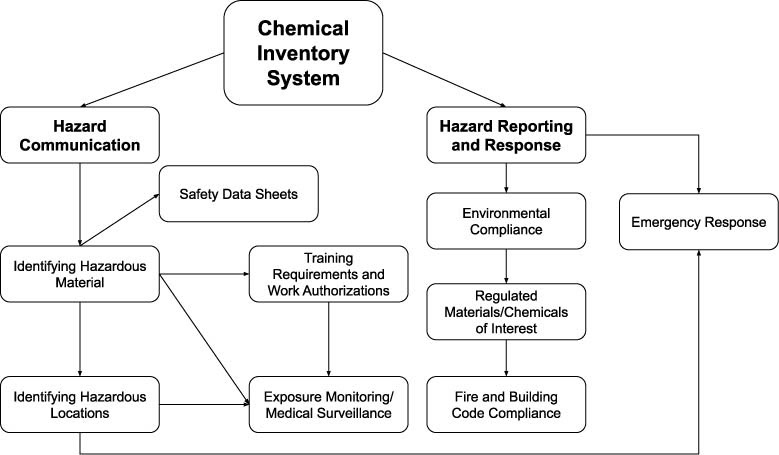
How the Chemical Inventory is Used
An accurate chemical inventory provides a number of utilities, such as those listed below.
- Efficient Ordering. An accurate inventory will help lab managers track the materials that are present in the laboratory and know whether additional supplies need to be ordered before duplicate/excess orders are placed.
- Locating reagents. Defining and logging locations where reagents are located make it easier and quicker for researchers to locate the reagents stored in the lab before orders are placed for materials already available in the lab.
- Deliberate disposal. An accurate inventory helps to identify materials that are no longer in use as well as tracking materials that have a limited shelf-life, including those that may become dangerous over time.
- Effective response. Chemical inventories provide valuable information to help fire and hazmat responders mount a safe and effective response to emergencies on campus.
- Compliance with hazardous material limits. Fire and building codes limit the amount of hazardous material that may be stored within a location based on a number of factors associated with the construction. The Department of Homeland Security has identified particular chemicals of interest which when stored above defined thresholds may require additional site security measures to be taken to protect against intentional release, theft, or sabotage. Accurate reporting of chemical inventories (both qualitatively and quantitatively) allow the life safety risk of hazardous material overages to be identified and remedied.
- Effective hazard communication. An accurate chemical inventory allows hazards associated with materials to be identified and communicated to potential users. The inventory also serves as a reference point to ensure comprehensive access to Safety Data Sheets.
- Safer storage practices. An accurate inventory can help to alleviate some of the work that can be associated with identifying areas where incompatible materials are being stored together and facilitate planning for how to utilize more effective and safe chemical storage and segregation practices.
General Considerations for Chemical Storage Locations
- Avoid storing materials on top of cabinets. Clearance from the ceiling must be 18 inches for sprinklered labs and 24 inches for not sprinklered.
- Chemicals should be readily accessible and to reduce accidents, materials should not be stored on shelves higher than 5 feet (~1.5m).
- Ensure container weight does not exceed the load rating of the shelves. Heavier items and larger containers should be stored on lower shelves.
- Wall mounted shelving is not recommended for chemical storage.
- Corrosive liquids and Particularly Hazardous Substances (PHS) shall be stored below eye level.
- Do not store chemicals in fume hoods.
- Provide adequate storage space for chemicals within your lab.
- Keep chemicals away from sources of heat or direct sunlight.
- Use secondary containment when possible.
- Use properly rated and labeled refrigerators and freezers when storing flammable materials.
Segregation
Chemicals should be stored with an effort to separate or segregate incompatible materials. This will serve to reduce the risk of mixing in case of accidental breakage, fire, seismic event, or response to a laboratory emergency. Even when containers are tightly closed, fugitive vapors can contribute to incompatibility reactions, leading to the creation of a hazardous condition or accelerate the degradation of labels, shelves, cabinets, and the containers themselves.
There are a variety of strategies used to effectively segregate incompatible chemicals. Common sense should be used when setting up storage areas so that workflow is not disrupted and to minimize travel between storage and use locations. Simply alphabetizing chemicals is no guarantee that incompatible materials will be effectively separated. Instead, one of the most common and effective strategies for storing chemicals utilizes a simple three-step approach:
- First materials are sorted by physical state (solids, liquids, and gases). Hazardous gases have particular storage requirements if they are stored outside of a gas cabinet or approved exhausted enclosure.
- Next materials are sorted from other incompatible materials. This is typically accomplished by grouping together materials with the same chemical reactivities (Chemical Storage Groups).
- Finally, the compatible materials within a Chemical Storage Group should be organized so that it will be easy to find and return containers. Organization approaches such as alphabetical (by name), carbon-length, and metal/counter-ion are commonly and effectively used.

