Gloves
- Glove Selection
- Glove Inspection Use and Care
- Proper Glove Removal
- Latex Gloves and Related Allergies
Wear gloves protect against skin absorption of chemicals, chemical burns, thermal burns, lacerations, and cryogenic liquid exposure. Choosing the appropriate hand protection can be a challenge in a laboratory setting.
Wear gloves when handling hazardous materials, chemicals of unknown toxicity, corrosive materials, rough or sharp-edged objects, and very hot or very cold materials. Disposable nitrile or neoprene gloves are usually appropriate as protection from incidental splashes or contact with lab chemicals. However, the SDS should be consulted to verify chemical compatibility with the gloves being used.
When working with chemicals with high acute toxicity, working with corrosives in high concentrations, handling chemicals for extended periods of time or immersing all or part of a hand into a chemical, the appropriate glove material should be selected, based on chemical and task compatibility.
Glove Selection
Consider the following when selecting a glove:
- Degradation rating
- Breakthrough time
- Permeation rate
Degradation is the change in the physical properties of a glove caused by contact with a chemical. Degradation typically appears as hardening, stiffening, swelling, shrinking or cracking of the glove. Degradation ratings indicate how well a glove will hold up when exposed to a chemical.
Breakthrough time is the elapsed time between the initial contact of the test chemical on the surface of the glove and the analytical detection of the chemical on the inside of the glove.
Permeation rate is the rate at which the test chemical passes through the glove material once breakthrough has occurred and equilibrium is reached. Permeation involves absorption of the chemical on the surface of the glove, diffusion through the glove, and desorption of the chemical on the inside of the glove. If chemical breakthrough does not occur, then permeation rate is not measured.
Manufacturers stress that permeation and degradation tests are done under laboratory test conditions, which can vary significantly from actual conditions in the work environment.
For mixtures, it is recommended that the glove material be selected based on the shortest breakthrough time.
The following table includes major glove types and their general uses. This list is not exhaustive.
| Glove Material | General Uses |
|---|---|
| Butyl | Offers the highest resistance to permeation by most gases and water vapor. Especially suitable for use with esters and ketones. |
| Neoprene | Provides moderate abrasion resistance but good tensile strength and heat resistance. Compatible with many acids, caustics and oils. |
| Nitrile | Excellent general duty glove. Provides protection from a wide variety of solvents, oils, petroleum products and some corrosives. Excellent resistance to cuts, snags, punctures and abrasions. |
| PVC | Provides excellent abrasion resistance and protection from most fats, acids, and petroleum hydrocarbons. |
| PVA | Highly impermeable to gases. Excellent protection from aromatic and chlorinated solvents. Cannot be used in water or water-based solutions. |
| Viton | Exceptional resistance to chlorinated and aromatic solvents. Good resistance to cuts and abrasions. |
| Silver Shield | Resists a wide variety of toxic and hazardous chemicals. Provides the highest level of overall chemical resistance. |
| Natural Rubber | Provides flexibility and resistance to a wide variety of acids, caustics, salts, detergents and alcohols. |
Compatibility Information
Most glove manufacturers have chemical compatibility charts available for their gloves. These charts may be found online from Fisher Scientific and Chemrest.
Most SDS recommend the most protective glove material in their Protective Equipment section. SDS's are available from the chemical manufacturers.
Other Considerations
Where fine dexterity is needed and can't be achieved with a manufactured product, consider double gloving with a less compatible material, immediately removing and replacing the outer glove if there are any signs of contamination. In some cases, such as when wearing Silver Shield gloves, it may be possible to wear a tight-fitting glove over the loose glove to increase dexterity.
Glove thickness, usually measured in mils or gauge, is another consideration. A 10-gauge glove is equivalent to 10 mils or 0.01 inches. Thinner, lighter gloves offer better touch sensitivity and flexibility, but may provide shorter breakthrough times. Generally, doubling the thickness of the glove quadruples the breakthrough time.
Glove length should be chosen based on the depth to which the arm will be immersed or where chemical splash is likely. Gloves longer than 14 inches provide extra protection against splash or immersion.
Glove size may also be important. One size does not fit all. Gloves which are too tight tend to cause fatigue, while gloves which are too loose will have loose finger ends which make work more difficult. The circumference of the hand, measured in inches, is roughly equivalent to the reported glove size. Glove color, cuff design, and lining should also be considered for some tasks.
Glove Inspection, Use and Care
Inspect gloves for signs of degradation or puncture before use. Test for pinholes by blowing or trapping air inside and rolling them out. Do not fill them with water, as this makes the gloves uncomfortable and may make it more difficult to detect a leak when wearing the glove.
Change disposable gloves when there is any sign of contamination. Reusable gloves should be washed frequently if used for an extended period of time.
Do not wear gloves outside of the laboratory. Utilize carts or carriers to transport research materials from the lab to other support areas. If materials must be hand-carried, utilize one gloved hand and one non-gloved hand to allow for touching common area objects; e.g., door knobs, elevator buttons, etc.
Be careful not to handle anything but the materials involved in the procedure while wearing gloves. Touching equipment, phones, wastebaskets or other surfaces may cause contamination. Resist touching your face, hair, and clothing as well.
Before removing them, wash the outside of the glove. To avoid accidental skin exposure, remove the first glove by grasping the cuff and peeling the glove off the hand so that the glove is inside out. Repeat this process with the second hand, touching the inside of the glove cuff, rather than the outside. Wash hands immediately with soap and water.
Follow the manufacturer’s instructions for washing and caring for reusable gloves.
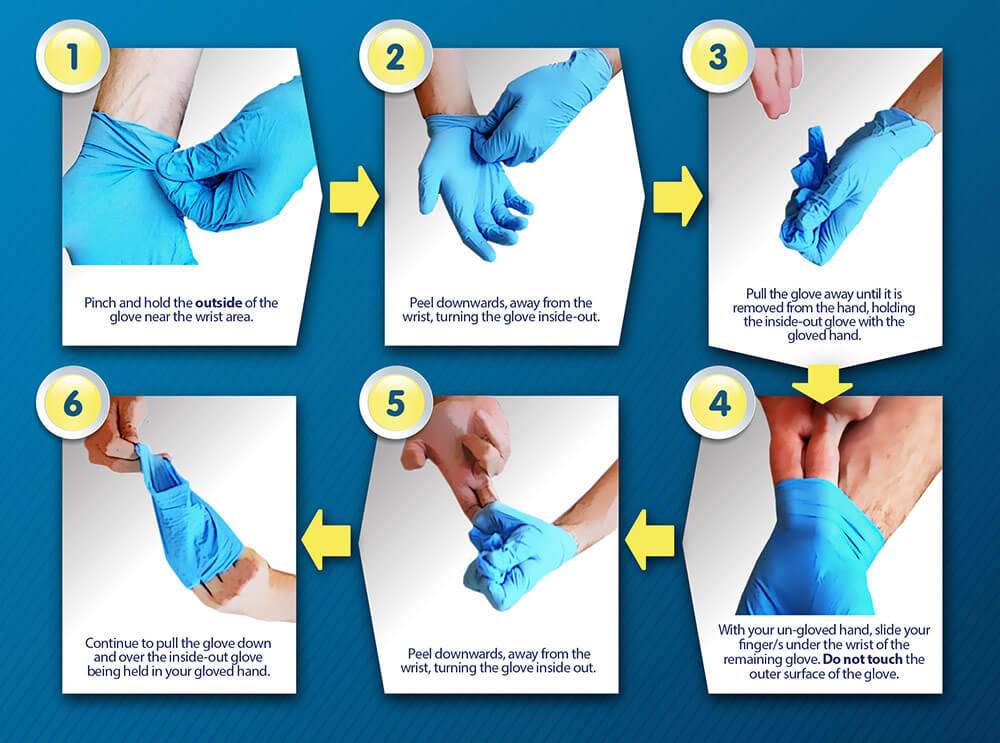
Proper Glove Removal
Gloves should be removed avoiding skin contact with the exterior of the glove and possible contamination. Disposable gloves should be removed as follows:
Latex Gloves and Related Allergies
Latex may cause sensitivity or become an allergen to those exposed. Latex exposure symptoms include skin rash and inflammation, respiratory irritation, asthma and shock. The amount of exposure needed to sensitize an individual to natural rubber latex is not known, but when exposures are reduced, sensitization decreases.
NIOSH recommends the following actions to reduce exposure to latex:
- Whenever possible, substitute another glove material.
- If latex gloves must be used, choose reduced-protein, powder-free latex gloves.
- Wash hands with mild soap and water after removing latex gloves.

