Step 3: Completing and Submitting the IRB Proposal Form
To conduct human subject research, researchers must complete and submit the university's IRB Proposal Form. The form, if properly completed, will help researchers to communicate their intended participants, recruitment procedures, research questions, and methodology, as well as the risks and benefits researchers see as potentially possible in/from the study's procedures. In addition, the IRB Proposal Form includes spaces to attach CITI Certificates (Step 1) and supporting documents, including an Informed Consent document (Step 2).
On this page, you will find a step-by-step guide for completing the IRB Proposal Form. This guide uses images of the IRB Proposal Form's elements, along with written descriptions and hints to assist researchers in the completion of the form.
Step-by-Step Guide to IRB Proposal Form
Page 1: Landing Page for Proposals
When you click on the proposal button/link, you'll 1) be asked to log in to mySUU if you are not already, and 2) arrive at the landing page for proposals in mySUU. All of your submitted proposals using the new system will be listed on this page. To create a proposal, you will click the green button that says "Create Proposal."

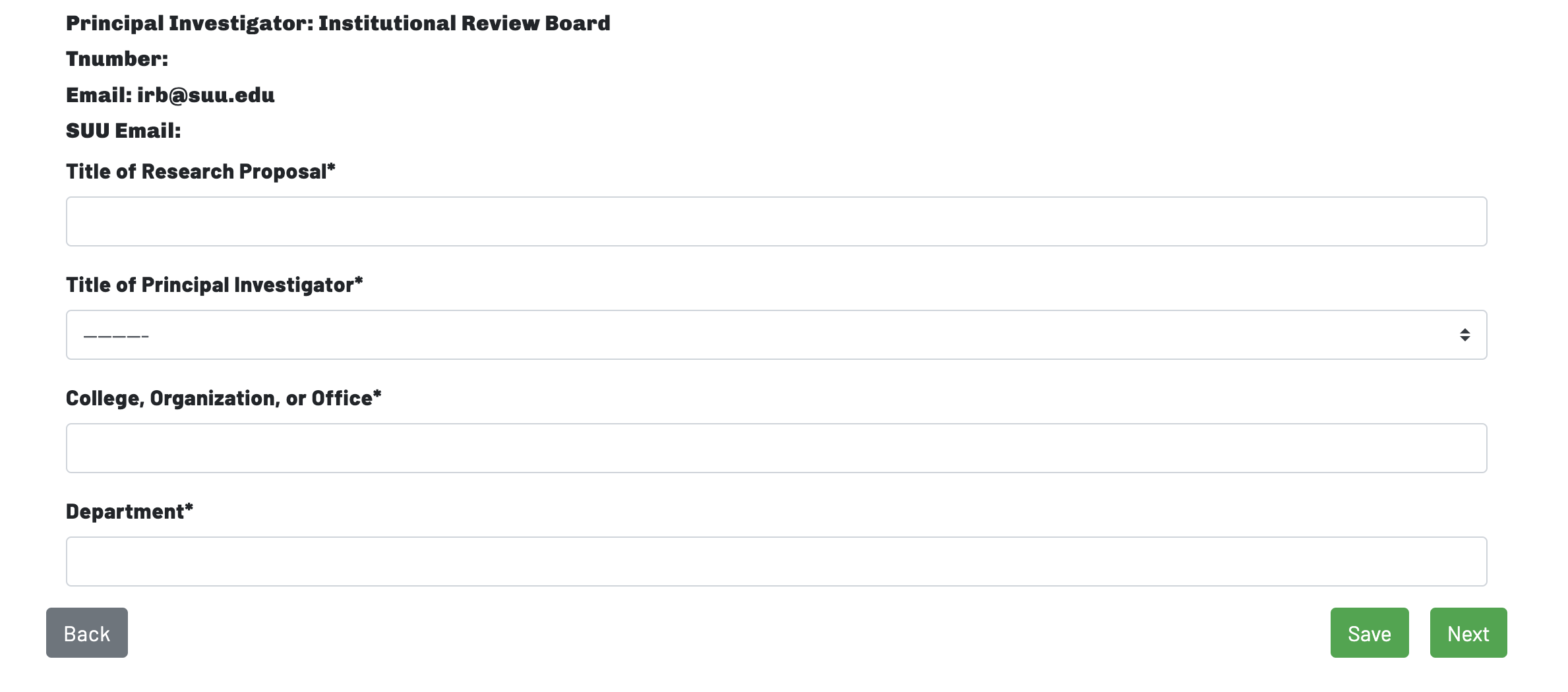
Page 2: Basic Information
On Page 2, the researcher will be asked to begin inputting basic information about the study. This basic information will include A) the title of the research proposal, B) the principal investigator's title (i.e., student, staff, faculty), C) the principal investigator's college, organization, or office, and D) the principal investigator's department.
Researchers who are not SUU students, staff, faculty, or affiliates should identify their home institution in the "College, Organization, or Office" and "Department" fields.
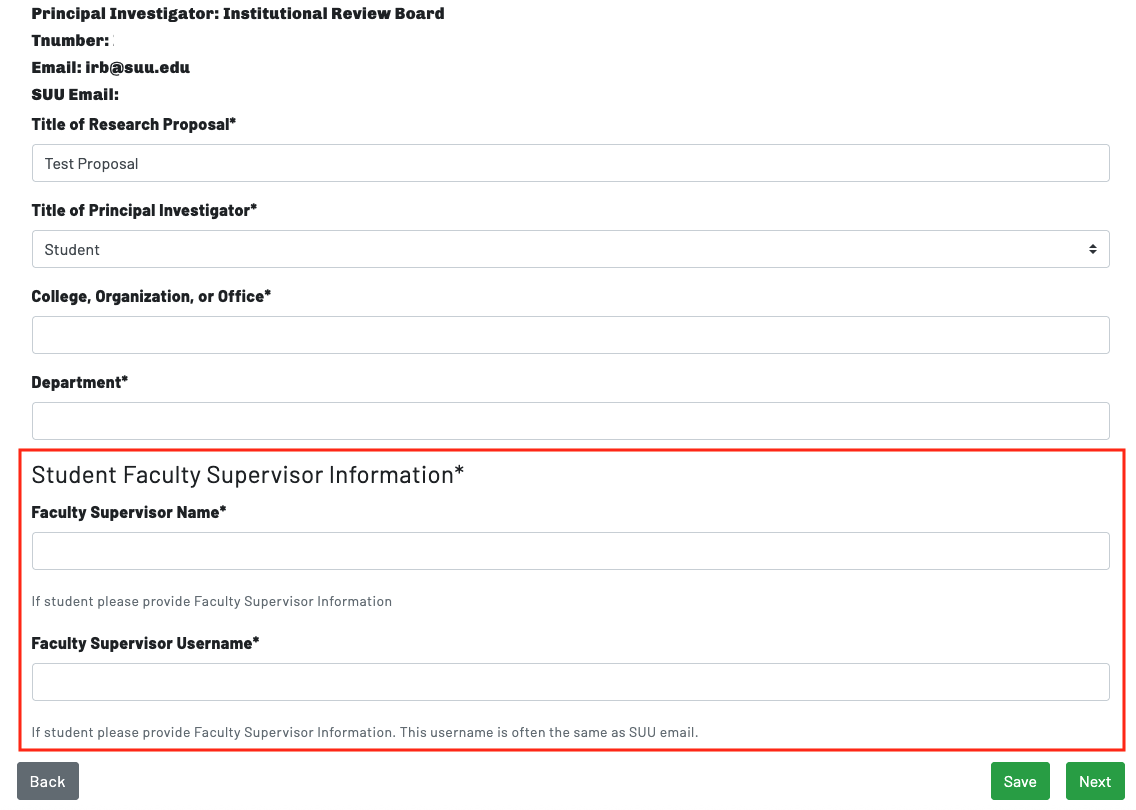
Page 2: Faculty Supervisor Info
If a principal investigator selects "Student" as role, additional prompts will appear to enter information regarding the faculty member supervising the project. All student researchers, both undergraduate and graduate, must have a faculty member supervising their research. This faculty member must be identified, and a current userid or email address for the faculty member must be provided; when you submit the IRB Proposal Form, the faculty supervisor will be prompted to review the research proposal. Failure to provide the requested information for faculty supervisors will delay the review and approval process.
Page 3: Other Investigators
List all investigators involved in the project, including faculty supervisors when applicable. Other investigators include any other person affiliated with the project who will 1) obtain consent from research participants and/or 2) collect and analyze data from research participants. Other investigators can be faculty, staff, and/or students. In addition, "classroom observers" in education graduate student research proposals should be identified as other investigators.
You can separate investigators in the box by using a semi-colon.
Write "N/A" in the box if there is no faculty supervisor or other investigators.
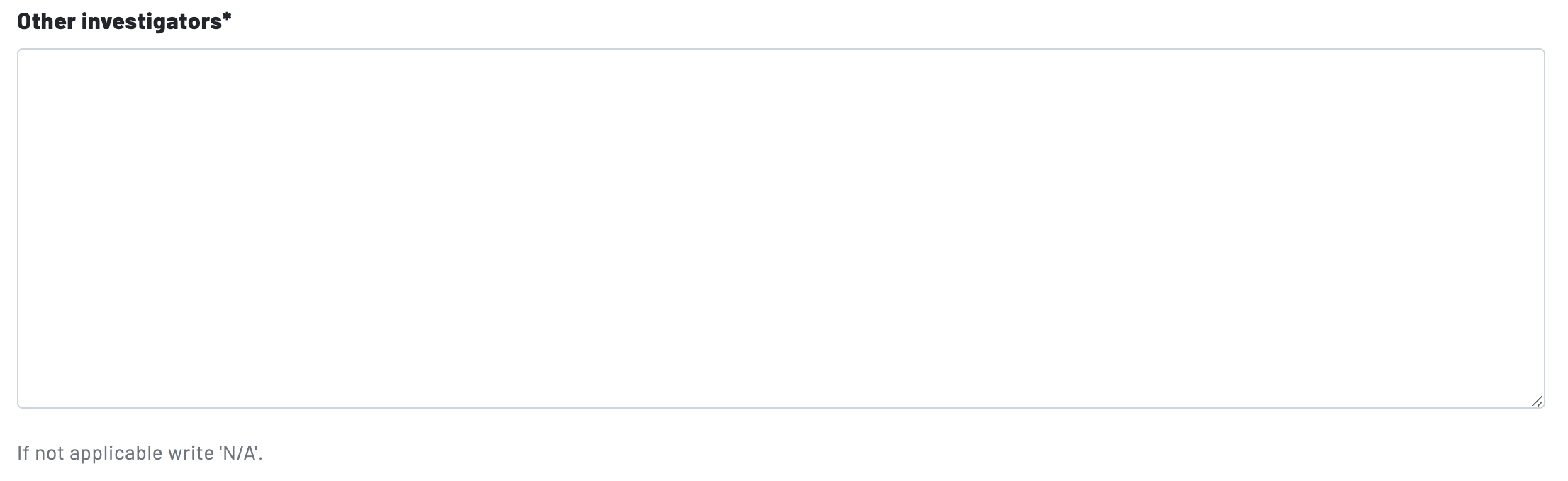
Page 3: CITI Certificates
The next part of Section 2 will offer a space to upload CITI certificates. A CITI certificate should be uploaded for each person identified as a researcher: the principal investigator, faculty supervisor (if applicable), and any other investigators. Failure to submit CITI certificates for all investigators in the study may result in review and approval delays.
To add a CITI certificate, click "Browse" and attach the relevant CITI certificate.
SUU affiliates (students, staff, and faculty) must complete the appropriate CITI training and submit a current certificate:
- Faculty and staff must complete the Faculty and Staff Researchers training.
- Undergraduate and graduate (except education) students will complete the Student Researchers training.
- All education graduate students (and any "classroom observers") will complete the Education Graduate Student Researchers training.
Note for Proposals with Non-SUU Researchers: Please attach CITI certificates and CITI score reports for any non-SUU researcher listed as an investigator for your study. The IRB will review score reports to compare completed modules with the CITI requirements for SUU affiliates

Page 3: Other Investigators' CITI Certificates
All researchers identified as investigators must complete the appropriate CITI training and submit a certificate. To add additional CITI certificates, click on the blue "Add IRB Training Certificate Document Upload" button, then attach the certificate. You can click the blue button multiple times to reveal multiple upload boxes.
Section 2: Start and End Dates
Page 3 will also ask for the start and end date of the study. The choice of dates is important. Participants cannot be recruited and data cannot be collected until the proposal is reviewed and approved by the SUU IRB, so recording a start date that is the same as the date the proposal is submitted does not offer any advantage. Similarly, the researcher must ensure that the identified end date for the study leaves sufficient time for the study to be completed.
Click on the grey box underneath "Start Date of Proposed Research" and "End Date of Proposed Research" to select start and end dates.
Note: IRB proposals approved as exempt or expedited usually do not expire, but if the study extends beyond the identified end date the researcher must submit a modification/addendum request to the IRB. More information can be found on the SUU IRB web site under "Managing Your Approved IRB Proposal."
Page 3: Purpose of Research
Next, the IRB Proposal Form will ask questions about the purpose of research and the course it is for (if applicable). Researchers should check all options that apply.
Page 3: Dissemination of Research
Next, the IRB Proposal Form will ask where the study's results will be disseminated. Once again, researchers should select all options that are relevant to the project.
Page 4: Research Justification and Questions
Page 4 asks that researchers identify their research questions and also provide a justification for their research. A few notes on this prompt might be helpful for researchers:
- Research questions are the questions that anchor or guide the study; in other words, the study procedures you identify later in the IRB Proposal Form should be designed to inform or answer the research questions. Research questions are generally not the word-for-word questions you might ask a research participant in the study.
- Your justification should be grounded in relevant academic literature for the area researchers intend to study. This prompt is the place to share the highlights of a literature review for the study, such as what a thesis student might produce for "Chapter 2" of a thesis. The reason researchers should draw on the key literature of their area is that the IRB member who conducts an initial review of your study may know little to nothing about your academic discipline and the gaps and problems of the literature.

Page 4: Participant Recruitment
Page 4 asks researchers to detail who the participants of the study will be and how they will be selected/recruited. The first set of prompts related to recruitment ask researchers to identify the estimated number of participants, as well as whether the participants are adults outside of the SUU community, minors, and/or SUU faculty, staff, or students.
Page 4: Recruitment Approaches and Compensation
Page 4's other prompts deal with participant recruitment. The most important of these prompts asks the researcher to describe how research subjects will be recruited, as well as any compensation/incentives provided for participation. The following is information to consider providing in this section:
- How will you recruit participants? What sources will you draw participants from?
- Is this group of participants a random or convenience sample of individuals?
- Is this group a purposive sample? If so, what characteristics, qualities, experiences, etc. will participants have in common?
- How old will participants be? Or what age range will they fall within?
- Education Graduate Student Researchers: What grade level(s) are participants?
- Education Graduate Student Researchers: What level of K-12 school are participants being recruited from?
IMPORTANT NOTE: THE USE OF INSTITUTIONAL AND/OR GRANT FUNDS TO COMPENSATE OR INCENTIVIZE PARTICIPANTS MUST BE DOCUMENTED IN THiS SECTION OF THE IRB PROPOSAL FORM.

Page 4: Recruitment Methods
Page 4 also includes a series of checkboxes requesting information concerning specific recruitment methods researchers might use. The checkbox utilizes an "other" option in the event the study employs recruitment methods other than those listed. Researchers should write in a description of these alternative recruitment methods.

Page 4: Recruitment Script or Flyer
The final item on Page 4 asks for a recruitment script. Recruitment scripts are texts or flyers that are used to communicate the study's purpose and particulars to potential research participants. The IRB Proposal Form provides the option of A) typing in the recruitment script, flyer, or text, or B) attaching a document produced outside of the IRB Proposal Form. When attaching recruitment scripts or flyers, write "N/A" into the text box.
Page 5: Materials and/or Apparatuses
Page 5 focuses on the research methodology used by researchers, beginning with materials and/or apparatuses. These are generally the data collection procedures of the study: surveys, interview/focus group questions, observation protocols, and exercises and exercise equipment, among others. In this space, researchers should:
- List and briefly describe the materials to which research participants will be exposed; if the materials are attached later in the IRB Proposal Form, a lengthy explanation in Page 5 is likely not necessary
- List and briefly describe the apparatuses (devices, equipment, machines, etc.) to which research participants will be exposed. Many studies may not utilize apparatuses.
- In Section 10 of the IRB Proposal Form, materials like surveys, interview/focus group questions/protocols, and others should be attached so they are submitted with the form to the IRB for review.
Page 5: Research Methodology
Page 5 other main prompt asks researchers to describe their research methodology. The IRB generally does not evaluate the effectiveness of the research methodology for a given study--this responsibility is assumed to belong to the researcher and faculty supervisor, where applicable--but the IRB does examine the specific procedures identified in proposals to ensure that the study does what it purports to do, along with evaluating the procedures for risks and benefits.
The research methodology prompt should include the following:
- Research Approach: qualitative, quantitative, mixed-method
- Research Tradition: action research, phenomenology, case study, etc.
- Study Procedures: outline how participants will use/experience the materials and/or apparatuses identified above
- Deception: deception involves withholding the truth and/or intentionally misleading research participants; the use of deception--along with researcher's efforts to debrief participants on the deception--must be described in detail, if utilized
- Recording: describe any uses of video/audio recording in your study
- Analysis Procedures
- For quantitative methods, outline the statistical approach you intend to use (e.g., descriptive statistics; correlational analysis)
- For qualitative methods, outline and describe your coding approach (or other approach to data analysis)
Education Graduate Student Researchers: if you collect/analyze student grades or test scores, you should describe the following:
- the assignments, tests, or other products that are graded
- whether the assignments, tests, or other products include student names and/or whether you will be able to link names to grades
If you analyze your students' grades, you will likely need to obtain written consent from parents/guardians, regardless of how you protect students' identities in your study.
Page 5: Audio and/or Video Recording
The final item on Page 5 asks researchers to indicate if audio and/or video recording is being used in the study. The IRB and research participants both must be aware of the use of any audio or video recording. Recording must also be noted in the Informed Consent document provided to research participants.
Page 6: Vulnerable Populations
Page 6 asks that researchers identify characteristics of vulnerable participants, along with the total number of potentially vulnerable participants they anticipate recruiting for the study. Recruiting participants from vulnerable populations is not necessarily forbidden, but when such participants are recruited the researcher may be asked to take special measures to communicate the study and its risks and benefits to participants, to utilize adapted data collection approaches, and other measures.
Education Graduate Student Researchers: Individuals with learning disabilities are among the categories in the checklist for vulnerable populations. Researchers conducting classroom research with their own students should identify students identified for special education supports as vulnerable populations. Another group that might be considered vulnerable is English Language Learners, whose language capacities--and whose family language use--may require special measures as briefly described above.


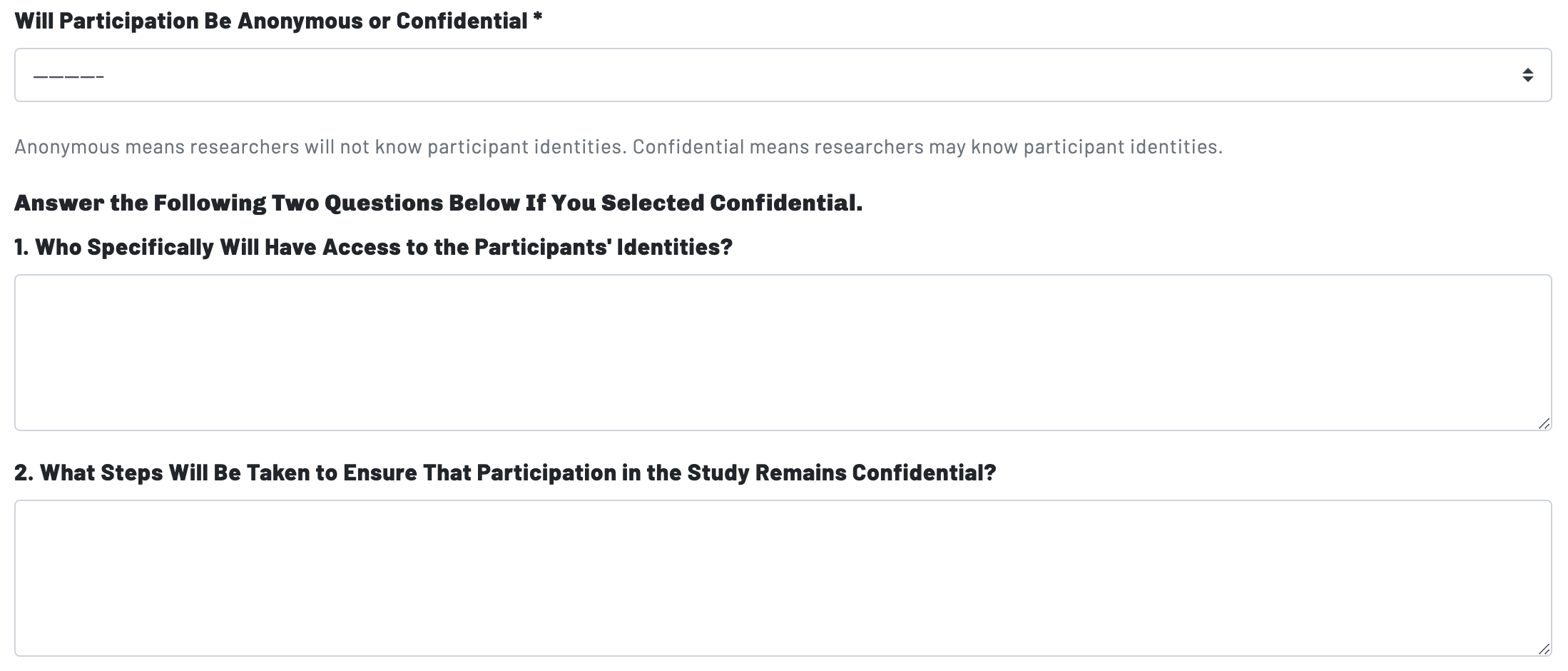
Page 7: Data Confidentiality & Anonymity
Section 6 includes two prompts regarding confidentiality and anonymity. In the first prompt, researchers are asked to identify whether their data collection will yield confidential or anonymous data. The difference between these two types of data is commonly mistaken in IRB Proposal Forms.
- Confidential (i.e., protected) data is data in which the researcher can link the data they collect to an individual participant. In these cases, the researcher knows--or can find out--who generated particular data points in the study but will ensure that no one outside of the research team will know which data source can be attributed to a particular participant. Researchers might use numbers, codes, or pseudonyms to identify data sources. For example, a teacher conducting classroom research of her own students might analyze student grades on a class roster, but protects students' individual identities by assigning each student a random four-digit number.
- Anonymous data is data in which the researcher cannot link data to an individual participant. The researcher will be totally unable to determine which participant generated a particular data point. For example, if a student surveys her peers online about their experiences in an Introduction to Biology course but does not collect names or any identifying information, her data would be anonymous.
In the first prompt of Page 7, researchers must also describe how they will protect participant data. Responses must include:
- Who will have access to the data? Identify all individuals who will have access to any research data, along with Informed Consent documents.
- What steps will be taken to ensure that data remains confidential? Such steps include using numbers, codes, or pseudonyms for participants. They may also include descriptions of how data will be stored (e.g., in a locked office and/or file cabinet; on a password or biometric-protected personal computer).
- When and how will this data be destroyed? Researchers must give the outline of a plan for the destruction or de-identification of any data. This plan might include a rough date for data destruction/de-identification, along with specific approaches to destroy data (e.g., shredding paper documents; permanently deleting files from a personal computer).

Page 7: Participant Confidentiality & Anonymity
Page 7's second prompt changes the focus from data to participants: in other words, the question becomes whether or not the researcher knows who the participants of the study are.
- Confidential participation means that the researcher may know--or be able to determine--the identity of a research participant and will take steps to ensure that no one outside of the research team knows that the study's participates were involved in the project. Researchers might take steps like minimizing the use of participant names or identifying information on materials other than the Informed Consent document, using codes, numbers, or pseudonyms to refer to participants and/or limiting contact regarding the study in the presence of other people (particularly non-participants).
- Anonymous participation means that the researcher will not know the identity of the research participants. In a study where participation is anonymous, the researcher may also refer to participants by codes, numbers, or pseudonyms, but regardless of how they refer to the participants they will not know who they actually are.
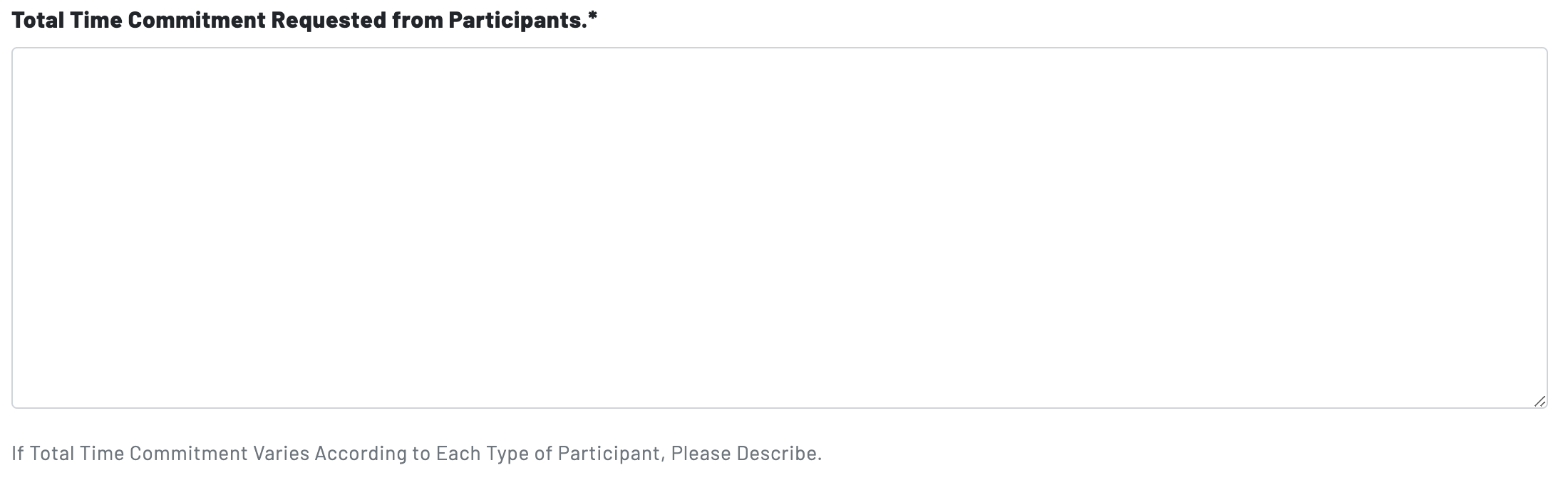
Page 8: Time Commitment
Page 8 begins with two prompts concerning the time commitment of participation in the study. The first prompt deals with the total time commitment for all participants, whereas the second prompt asks researchers to identify differences in time commitment between types or groups of participants.
- For the total commitment requested, researchers should answer in hours and weeks/months. For example: Participation will require approximately 10 hours over a 4 week period.
- For the time commitment according to participant types, researchers also should indicate hours and weeks/months, but broken down by each group of participants. If the time commitment is roughly the same for all participants, write "N/A" in the second prompt.
Section 7: Risks & Risk Mitigation
Section 7 continues with two prompts concerning information that will be solicited from participants that could potentially put the participant at physical, physiological, social, economic, and/or legal risk. Researchers' responses across these two prompts have important implications for both the mitigation of risk for research participants, as well as the review pathways the IRB can utilize in approving the study.
- In the first prompt, researchers will check all types of information that will be solicited from participants as part of the study.
- In the second prompt, researchers must describe how they will minimize the risks they have identified. For example, if the researcher intends to ask a research participant questions about their coworkers, the researcher should identify steps that will be taken to avoid risk(s) resulting from these responses; such steps could include--but are not limited to--holding an interview away from the workplace and coworkers, using codes or pseudonyms to mask the identities of individuals who are referenced, and/or declining to audio-record an interview if participants are uncomfortable with a record of their conversation existing due to the possible sensitivity of their contributions.


Page 8: Special Procedures
Page 8 continues by prompting researchers to indicate whether they will use special procedures. These procedures are important to accurately identify as they are used to determine the review category the IRB can use to approve the proposal. Procedures include medical procedures (e.g., blood sampling), moderate or strenuous exercise, procedures that could cause physical and/or psychological pain/discomfort, and others.
Most studies do not use these procedures. If the procedures are not used, "None of These Procedures Will Be Utilized" should be selected.

Page 8: Benefits
Page 8 next asks about the study's possible benefits. Benefits are advantages gained from participation in the study's procedures. For example, if a researcher wanted to test the effectiveness of a new supplement, a possible benefit might be the advantage provided by the supplement itself (i.e., perhaps the supplement benefits a person's health in some way).
Benefits are different than compensation. Benefits come from participation in the study's procedures. Compensation is an enticement to participate or to complete participation in the study's procedures. For example, if the researcher in the example above gave a $25 gift card to anyone who completed the study involving the supplements, this would be an example of compensation.
A few notes on benefits:
- Researchers must take care not to overstate or guarantee the possible benefits of a study. There is an important difference between saying that a participant may benefit from a study versus saying they will benefit from its procedures.
- Most studies do not result in benefits for a participant. There is nothing wrong with a research study that has no direct benefit to research participants.
- Researchers must write "There is no direct benefit to participation" in the IRB Proposal Form and in the Informed Consent document when no benefits are possible from a study.
- Researchers may identify possible benefits to individuals, organizations, academic disciplines, and others in their proposal, but this should always come after a clear statement of the study's benefits (or lack of benefits) for participants themselves.

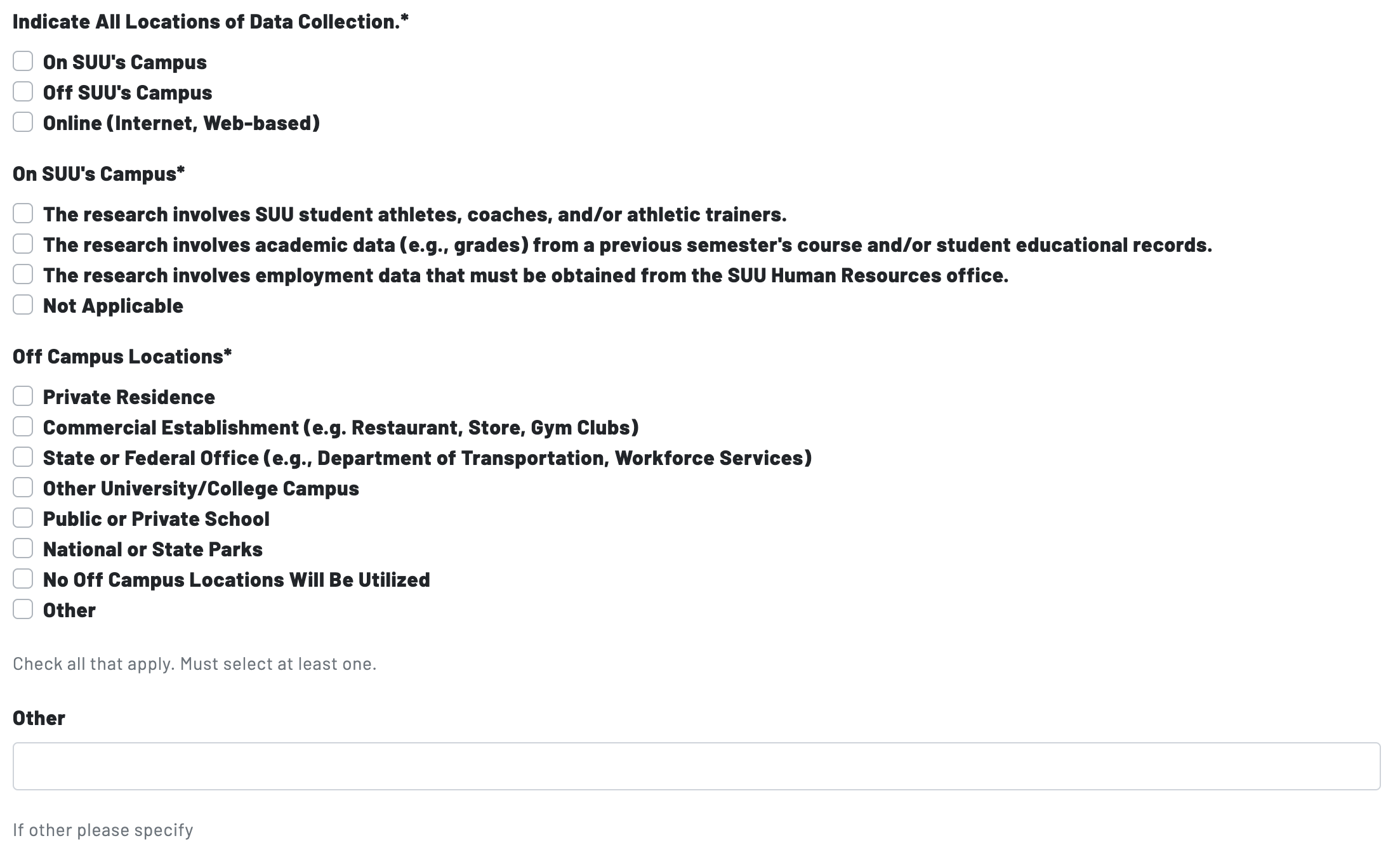
Page 8: Research Locations
Page 8 concludes with two prompts concerning the locations of data collection. Researchers will select whether they are collecting data on campus, off campus, and/or online, then identify particular locations whether data collection is taking place.
Researchers may be required to secure permission to obtain consent and collect data from off campus locations. The IRB can grant permission for research activities that take place on campus, as well as many instances where research is conducted in public settings. However, for research conducted in non-public settings--or settings deemed as non-public for the purposes of research--permission from a individual with authority at the intended research site is necessary.
To secure permission, researchers will be asked to secure a letter of permission or "site letter." See the Step 2: Supporting Documents guide for additional information and requirements.
Examples of Non-Public Settings: K-12 schools; universities other than SUU; religious services; governmental offices; workplaces; commercial establishments

Page 9: Informed Consent
Page 9 focuses on the Informed Consent Documents. Typically, researchers are expected to obtain written consent from research participants. Virtually all research participants must be provided with information on the elements of informed consent prior to participating in a survey: the purpose of the study; consent and withdrawal procedures; confidentiality; study procedures; duration of participation; risks; benefits; and compensation.
To complete the first prompt, researchers should consider the following questions:
- Are the study's data collection procedures face-to-face? If data collection procedures are conducted face-to-face, researchers should plan to present the Informed Consent Document and obtain/safeguard written consent.
- Are the study's data collection procedures online or asynchronous? In these cases, researchers may obtain consent by embedding an Informed Consent Document or statement in their data collection measures (e.g., a survey) and requiring participants to indicate their consent by answering a yes or no question
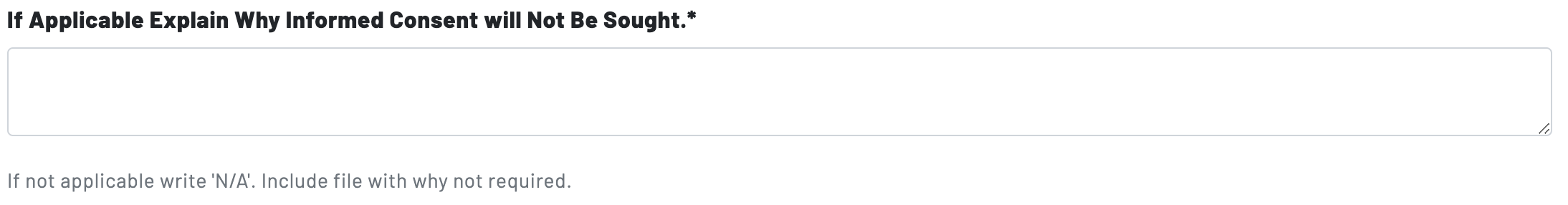
In the event informed consent will not be sought, researchers must justify their reason(s) for an exception to the informed consent requirement. The IRB ultimately will determine whether the informed consent requirement can be waived.
The next prompt asks researchers to identify if a separate script is used to recruit participants. The script might be understood as a "recruitment pitch" for participants, which will presumably describe many of the study's elements (including elements of informed consent). If a separate script is not used--and researchers simply read/present the Informed Consent Document, this prompt can be responded to with "N/A."

Page 9: Informed Consent Checklist & Upload
Page 9 concludes with a checklist of required elements for Informed Consent Documents, along with a box for the researcher to upload the study's Informed Consent Document. The elements of informed consent identified in this list are also included in the informed consent templates located on the IRB's web site. See the Step 2: Supporting Documents guide for consent templates, an annotated consent form, and other tips for drafting Informed Consent Documents.
Note: The IRB will request revisions to Informed Consent Documents when they do not include the elements of informed consent.


Page 10: Funding
Page 10 includes two prompts concerning funding. Most studies the IRB reviews are self-funded or funded by entities within the university community. Human subject research studies funded beyond the university community through grants and other funding mechanisms are regulated both by the IRB, as well as the Sponsored Programs, Agreements, Research and Contracts (or SPARC) office.
BEFORE COMPLETING AND SUBMITTING AN IRB PROPOSAL, RESEARCHERS MUST COMPLETE ALL SPARC PRE-AWARD REQUIREMENTS. See the Funded Research page for more information on the SPARC and IRB approval processes.
Page 11: Supporting Documents
Page 11 is where researchers upload all supporting documents--excluding CITI certificates and Informed Consent Documents--for their study. The upload box permits the upload of multiple documents. Documents identified as "materials" on Page 4's "Materials and Apparatuses" prompt should be uploaded here, if they were not uploaded as a single document on Page 4.
See the Step 2: Supporting Documents page for more information on developing supporting documents.

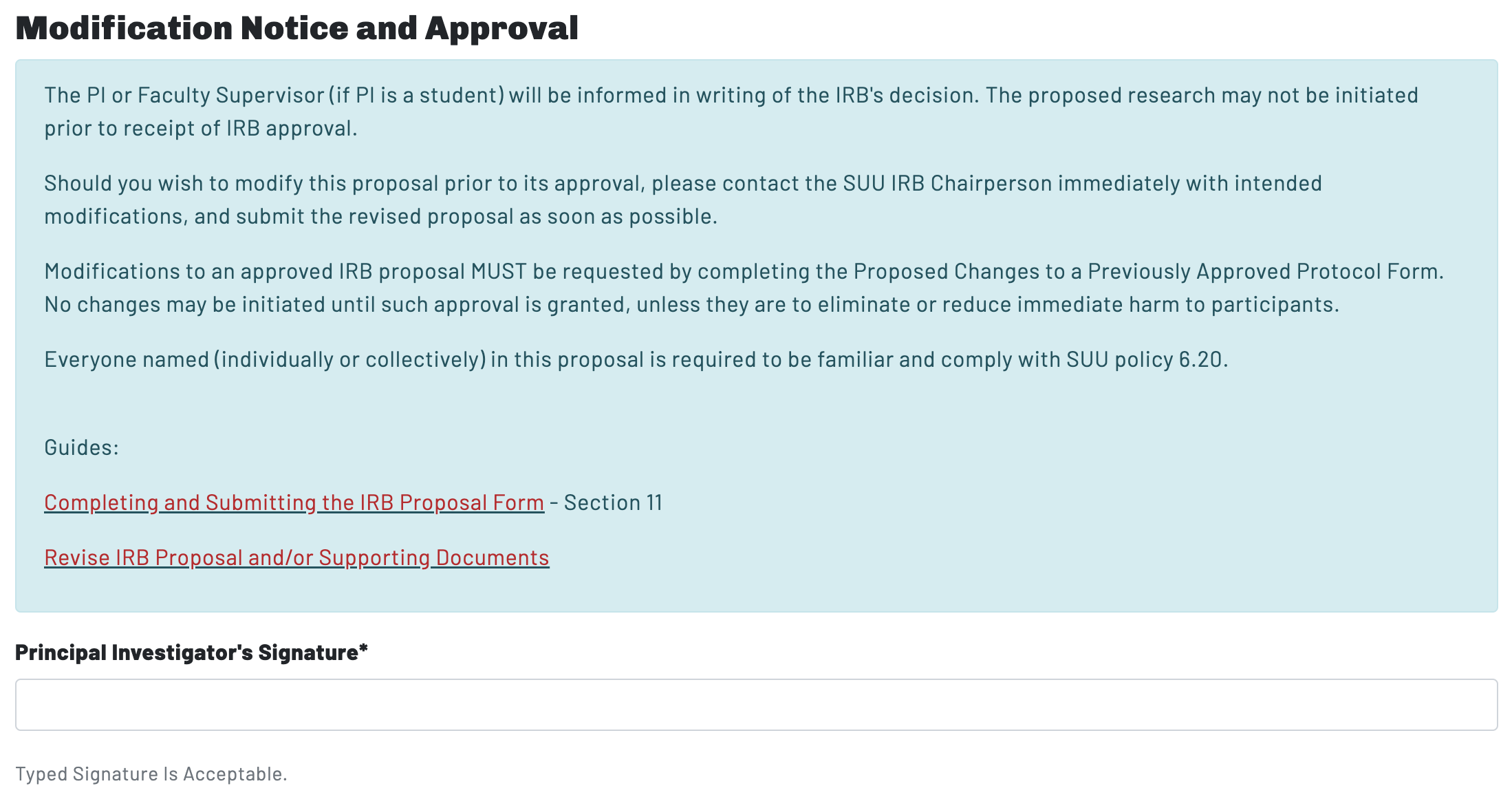
Page 12: Modification Notice & Approval
Page 12 starts with a statement about modifications. Once approved, your IRB proposal sets the boundaries for your study; the approved proposal is a protocol researchers are expected to use and by which they should abide. If researchers desire to change their study's procedures or elements after approval, they must complete the IRB Proposal Modification/Addendum form.
To finish and submit the proposal, the researcher should sign and date the form and click submit. Once submitted, the IRB Proposal Form will be sent to the IRB and to faculty supervisors (if applicable, and assuming the faculty supervisor's userid was properly inputted into the form).